Everything in our universe - the air we breathe, the food we eat, the chair we sit on, and even the stars in the sky - is made up of matter. This NCERT Class 9 Science chapter is all about mass and volume and how it can exist in different forms.
For this chapter, we have covered the physical nature of matter, characteristics of particles of matter, the states of matter (solid, liquid, gas), how matter changes state due to temperature and pressure, along with everyday real-life applications.
Notes & Study Material PDF
Check out all the topics examinable this year for the final exams from CBSE Class 9 Syllabus.
Physical Nature of Matter
Early Indian philosophers described it as the “Panch Tatva” (air, water, fire, sky, and earth). Modern science confirms matter is made up of tiny particles. So matter is literally anything and everything around us.
Now the question is how small are these particles? We can understand this by examples.
- When you dissolve sugar in water, it slowly disappears. How? Sugar particles took spaces between water particles.
- Another example could be a few crystals of potassium permanganate that can color 1000 L of water. This shows particles are extremely small and divisible.
Characteristics of Particles of Matter
Particles of matter show certain fundamental properties that explain how solids, liquids, and gases behave. Let’s look at some of them.
- Particles have space between them. For example, when sugar, salt, or Dettol is added to water, the level of water does not rise. This is because the particles of these substances occupy the empty spaces between water particles.
- They are continuously moving. For example, the smell of hot cooked food reaches far away faster than cold food because gas particles, at a high temperature, have increased kinetic energy and hence, faster speed. Other examples are the diffusion of perfume in a room.
- All these particles attract each other. However, the strength of force varies - very strong in solids, weaker in liquids, and weakest in gases. For example, liquids flow but don’t escape easily, and wooden blocks that are hard to cut.
States of Matter
Primarily there are three states of matter - Solid, Liquid, and Gas. Here is a quick recap on their properties.
1. The Solid State
Solids usually have fixed shape, volume, and distinct boundaries. For example: ice, wood, chalk, etc. But there are some exceptions like rubber band - is different due to its elasticity, sponge - is compressible due to air pores, sugar/ salt - they take container shape but individual crystals remain solid.
2. The Liquid State
Liquids have fixed volume but no fixed shape. They flow easily and that’s why they are also called fluids. In comparison to solids, liquids can diffuse/ dissolve faster. Some examples are water, milk, and oil.
3. The Gaseous State
Gases are the most free form of matter. They have no fixed shape or volume, are highly compressible, for example CNG, LPG. Again, when compared with solids & liquids, gases are the fastest to diffuse into each other. This is mainly due to large intermolecular spaces.
Change of State of Matter
An interesting thing about matter is that it can transform its states under certain conditions.
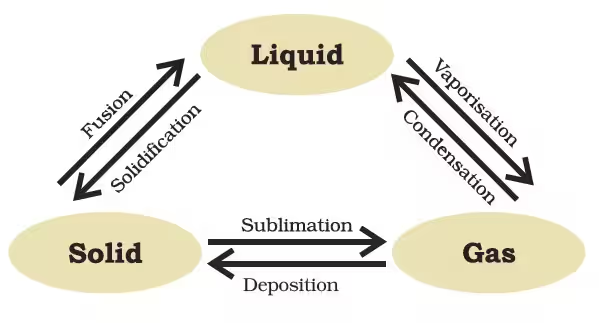
Go through the examples given below to understand how.
1. Effect of Temperature
Whenever the temperature of a particle is increased, it induces a change in its kinetic energy. This brings about a change in their current intermolecular forces and hence, their state. For example:
- Ice melts at 273 K (0°C) - solid is transforming to liquid
- Water boils at 373 K (100°C) - liquid starts evaporating, i.e., transforms to gas
Latent Heat
Latent heat is the amount of hidden heat energy required to change the state of matter without changing its temperature. There are two categories of latent heat.
- Latent Heat of Fusion - Heat required to convert 1 kg of solid into liquid at its melting point without rising its temperature. Example: Ice at 0°C absorbs latent heat while melting into water at 0°C.
- Latent Heat of Vaporisation - Heat required to convert 1 kg of liquid into vapour at its boiling point without any rise in temperature. Example: Water at 100°C absorbs latent heat while changing to steam at 100°C.
Note: Steam at 100°C has more energy than water at 100°C because of the latent heat of vaporisation. This is why steam burns are more severe than boiling water burns.
2. Effect of Pressure
Whenever the pressure is applied and temperature is reduced, gases can be liquefied. For example:
- LPG (Liquified Petroleum Gas) and CNG (Compressed Natural Gas) are stored under high pressure.
- Dry ice is actually solid CO₂ (Carbon Dioxide) allowed to sublimate directly to gas. It does not pass through a liquid state, which is why it is called dry ice.
Evaporation and Cooling
Evaporation is the slow process by which liquid particles at the surface gain enough energy to escape into vapour, even below the boiling point.
Factors Affecting Evaporation
- Surface Area: Larger the surface area, faster the evaporation, for example, clothes dry faster when spread out.
- Temperature: As the temperature increases, more particles gain energy to escape.
- Humidity: Lower the humidity, faster evaporation, for example, clothes dry slower on humid days.
- Wind Speed: Faster winds help in quicker evaporation, for example, clothes dry quickly on a windy day.
Cooling Effect of Evaporation
Evaporation requires heat energy. Liquid particles absorb this energy from their surroundings and evaporate, leaving the surroundings cooler. For example:
- Sweat absorbs heat from the body and evaporates, keeping us cool.
- Sprinkling water on rooftops cools the house in summer.
- Nail polish remover or petrol on the skin feels cool because of rapid evaporation.
Everyday Applications
To wrap this up, let’s revise the chapter with the help of some real-life examples.
- Why ice at 0°C cools better than water at 0°C: Ice absorbs extra latent heat to melt, giving a stronger cooling effect.
- Why steam burns more than boiling water: Steam contains latent heat of vaporisation, so it releases more energy on condensation.
- Why camphor and naphthalene disappear on their own: They undergo sublimation (solid → gas).
- Why we see water droplets outside a cold glass: Water vapour in air condenses on the cold surface.
- Why cotton clothes are best in summer: Cotton absorbs sweat and allows evaporation, which cools the body.
- Why desert coolers work better on hot, dry days: Lower humidity increases the rate of evaporation, providing stronger cooling.
- Why LPG and CNG are supplied in cylinders: They are compressed into liquid state under high pressure, saving space and making transport easier.
Note: These can be asked as “Give Reason Why” Questions. Check out CBSE Class 9 Previous Year Papers for such new pattern Qs.
Conclusion and Key Takeaways
The chapter Matter in Our Surroundings introduces the concept that matter is made of tiny particles with distinct properties. By understanding their behavior under different conditions, we learn why matter exists in various states and how natural processes like evaporation, sublimation, and condensation occur around us.
From boiling water to LPG cylinders and from dry ice to sweat cooling our body, this chapter shows the practical relevance of simple scientific principles in daily life.
Key Takeaways
- Matter is made of small particles with space, motion, and attraction.
- States of matter differ in rigidity, compressibility, diffusion, and density.
- State change is possible by altering temperature or pressure.
- Sublimation and deposition are special state changes.
- Evaporation is a surface phenomenon and causes cooling.
FAQs
Q1. What are the characteristics of particles of matter?
Ans: Particles of matter have spaces between them, are continuously moving, and attract each other.
Q2. Why is ice at 0°C more effective in cooling than water at 0°C?
Ans: Ice requires latent heat of fusion to melt, absorbing extra energy from surroundings.
Q3. What is sublimation? Give examples.
Ans. Direct change of solid to gas without becoming liquid. Example: Naphthalene, camphor, dry ice.
Q4. Why does evaporation cause cooling?
Ans. During evaporation, particles absorb heat from surroundings to change into vapour, leaving the surroundings cooler.
Q5. Why should we wear cotton clothes in summer?
Ans. Cotton absorbs sweat and exposes it to air for faster evaporation, keeping the body cool.

.svg)










.avif)











