Students who have opted for science in Classes 11 and 12 can understand the stress of understanding the reactions, equations, and formulas in organic chemistry. This part of chemistry is really significant for entrance exams like NEET and JEE. Career options in the fields of biochemistry, medicine, pharmacy, and many others can be pursued after having a strong grasp of organic chemistry both theoretically and practically.
The Class 12 Organic Chemistry in 2023-24 CBSE Chemistry examinations comprise almost 33 marks. As the board exams are nearing, students can be seen to find the easiest way to complete the entire syllabus and score high marks. The formulas and reaction sheets given by Educart are created after extensive research and can help students handle the complexity of the subject. Integrate this formula sheet into the exam strategy and boost your preparation.
Download the complete CBSE Class 12 Organic Chemistry Formula.
List of Chapter-wise Organic Chemistry Formulas and Reactions
The branch that focuses on carbon-containing compounds is organic chemistry. Carbon with the help of different bond patterns can form distinct various and complex molecules. To get a better understanding of organic compound’s structure, behavior, properties, reactions, and synthesis, organic chemistry is important.
These are the chapters included in the organic chemistry CBSE syllabus for Class 12.
Unit VI: Haloalkanes and Haloarenes
Nomenclature




Classification
On the Basis of Number of Halogen Atoms

Compounds Containing sp3 C—X Bond (X= F, Cl, Br, I)
(a) Alkyl halides or haloalkanes (R—X)

(b) Allylic halides

(c) Benzylic halides

Compounds Containing sp2 C—X Bond
(a) Vinylic halides

(b) Aryl halides

Nature of C-X Bond


Preparation of Haloalkanes and Haloarenes
- From Alcohols

- From alkanes by free radical halogenation

- From Alkenes
- Addition of hydrogen halides:

In the case of unsymmetrical alkenes; Markovnikov’s Rule:

- Addition of halogens:

Halogen Exchange

- From hydrocarbons by electrophilic substitution

(ii) From amines by Sandmeyer’s reaction

Replacement of the diazonium group by iodine does not require the presence of cuprous halide and is done simply by shaking the diazonium salt with potassium iodide.

Physical Properties

The boiling points of isomeric haloalkanes decrease with increase in branching. For example, 2-bromo-2-methylpropane has the lowest boiling point among the three isomers.


Chemical Reactions
Reactions of Haloalkanes
(1) Nucleophilic substitution reactions



Groups like cyanides and nitrites possess two nucleophilic centres and are called ambident nucleophiles.
(a) Substitution nucleophilic bimolecular (SN2)

Spatial arrangement of functional groups around carbon is called its configuration. See the structures (A) and (B) given below carefully.

These are the two structures of the same compound. They differ in spacial arrangement of functional groups attached to carbon. Structure (A) is mirror image of Structure (B). We say configuration of carbon in structure (A) is mirror image of the configuration of carbon in structure (B).
the order of reactivity followed is: Primary halide > Secondary halide > Tertiary halide.

(b) Substitution nucleophilic unimolecular (SN1)


For the same reasons, allylic and benzylic halides show high reactivity towards the SN1 reaction. The carbocation thus formed gets stabilised through resonance as shown below:

For a given alkyl group, the reactivity of the halide, R-X, follows the same order in both the mechanisms R–I> R–Br>R–Cl>>R–F.
(c) Stereochemical aspects of nucleophilic substitution reactions


(iii) Retention:

(iv) Inversion, retention and racemisation:

When (–)-2-bromooctane is allowed to react with sodium hydroxide, (+)-octan-2-ol is formed with the –OH group occupying the position opposite to what bromide had occupied.


Elimination reactions

2-bromopentane gives pent-2-ene as the major product.

Reaction with metals

In the Grignard reagent, the carbon-magnesium bond is covalent but highly polar, with carbon pulling electrons from electropositive magnesium; the magnesium halogen bond is essentially ionic.

Grignard reagents are highly reactive and react with any source of proton to give hydrocarbons. Even water, alcohols, amines are sufficiently acidic to convert them to corresponding hydrocarbons.

Wurtz reaction

Nucleophilic substitution

Difference in hybridisation of carbon atom in C—X bond:

Replacement by hydroxyl group

The presence of an electron withdrawing group (-NO2) at ortho- and para-positions increases the reactivity of haloarenes.

Mechanism of the reaction is as depicted:

2. Electrophilic substitution reactions

(i) Halogenation

(ii) Nitration

(iv) Friedel-Crafts reaction

3. Reaction with metals
Wurtz-Fittig reaction

Fittig reaction

Trichloromethane (Chloroform)

Swarts reaction.

p,p’-Dichlorodiphenyltrichloroethane(DDT)

Unit VII: Alcohols, Phenols, and Ethers
Classification of Alcohols
- Based on the Number of Hydroxyl Groups
- > Monohydric alcohols: They contain one –OH group. Example, CH3CH2–OH
- Compounds Containing sp3 Hybridised carbon —OH bond
Primary alcohols: One or no carbon atom is directly attached.
Secondary alcohols: Two carbon atoms are directly attached.
Tertiary alcohols: Three carbon atoms are directly attached.

Allylic alcohols: In these alcohols, the —OH group is attached to a sp3 hybridised carbon adjacent to the carbon-carbon double bond, that is to an allylic carbon. For example

Benzylic alcohols: In these alcohols, the —OH group is attached to a sp3—hybridised carbon atom next to an aromatic ring. For example.

- Compounds Containing sp2 Hybridised carbon —OH bond
Vinylic alcohol: CH2 = CH–OH
->Dihydric alcohols: They contain two –OH groups. Example, 1, 2-Ethanediol.
-> Trihydric alcohols: They contain three –OH groups. Example 1, 2, 3-Propanetriol.

Nomenclature of Alcohols


Cyclic alcohols are named using the prefix cyclo and considering the —OH group attached to C–1.

Nomenclature of Phenols
Phenol is hydroxybenzene. Phenol is a common name for the compound. Its IUPAC name would be benzenol. Substituents are always numbered with the –OH group being given the first position in the alcohol chemistry formula.

Common name IUPAC name

Dihydroxy derivatives of benzene are known as 1, 2-, 1, 3- and 1, 4-benzenediol.

Ethers
Ethers are classified as simple or symmetrical, if the alkyl or aryl groups attached to the oxygen atom are the same, and mixed or unsymmetrical if the two groups are different. Diethyl ether, C2H5OC2H5, is a symmetrical ether whereas C2H5OCH3 and C2H5OC6H5 are unsymmetrical ethers.
Nomenclature of Ethers


Structures of Functional Groups

Preparation of Alcohols
- From Alkenes
- By Acid Catalysed Hydration

Mechanism
Protonation of alkene to form carbocation by electrophilic attack of H3O+.

Nucleophilic attack of water on carbocation.

Deprotonation to form an alcohol.

- By Hydroboration-Oxidation

- From Grignard Reagents


By Reduction of Carbonyl Compounds (Aldehydes and Ketones)

By Reduction of Carboxylic Acids and Esters

However, LiAlH4 is an expensive reagent, and therefore, used for preparing special chemicals only. Commercially, acids are reduced to alcohols by converting them to the esters, followed by their reduction using hydrogen in the presence of catalyst (catalytic hydrogenation).

Preparation of Phenols
From haloarenes

From benzenesulphonic acid

From Diazonium Salt

From Cumene

Physical Properties of Alcohol
The boiling points of alcohols and phenols increase with increase in the number of carbon atoms (increase in van der Waals forces). In alcohols, the boiling points decrease with an increase of branching in carbon chain (because of decrease in van der Waals forces with decrease in surface area). The –OH group in alcohols and phenols is involved in intermolecular hydrogen bonding as shown below:

For example, ethanol and propane have comparable molecular masses but their boiling points differ widely. The boiling point of methoxymethane is intermediate of the two boiling points.

The solubility of alcohols and phenols in water is due to their ability to form hydrogen bonds with water molecules as shown. The solubility decreases with an increase in the size of alkyl/aryl (hydrophobic) groups. Several of the lower molecular mass alcohols are miscible with water in all proportions.

Chemical Properties of Alcohols
- Alcohols as Nucleophiles

The bond between C–O is broken when they react as electrophiles. Protonated alcohols react in this manner.

Based on the cleavage of O–H and C–O bonds, the reactions of alcohols and phenols may be divided into two groups:
- Reactions of Alcohols Involving Cleavage of –OH bond
Acidity of Alcohols
- Reaction with metals:

In addition to this, phenols react with aqueous sodium hydroxide to form sodium phenoxides.

The above reactions show that alcohols and phenols are acidic in nature. In fact, alcohols and phenols are Brönsted acids i.e., they can donate a proton to a stronger base (B:).

- Acidity of alcohols
The acidic character of alcohols is due to the polar nature of the O–H bond. An electron-releasing group (–CH3, –C2H5) increases electron density on oxygen tending to decrease the polarity of the O-H bond. This decreases the acid strength. For this reason, the acid strength of alcohols decreases in the following order:

Alcohols are, however, weaker acids than water. This can be illustrated by the reaction of water with an alkoxide.

- Acidity of phenols:

The ionisation of an alcohol and a phenol takes place as follows:

Due to the higher electronegativity of sp2 hybridised carbon of phenol to which –OH is attached, electron density decreases on oxygen. This increases the polarity of the O–H bond and results in an increase in the ionisation of phenols than that of alcohols. Now let us examine the stabilities of alkoxide and phenoxide ions. In alkoxide ion, the negative charge is localised on oxygen while in phenoxide ion, the charge is delocalised. The delocalisation of negative charge (structures I-V) makes phenoxide ions more stable and favours the ionisation of phenol.
Although there is also charge delocalisation in phenol, its resonance structures have charge separation due to which the phenol molecule is less stable than the phenoxide ion.

The greater the pKa value, the weaker the acid.

- Reaction with carboxylic acid (Esterification):

The introduction of acetyl (CH3CO) group in alcohols or phenols is known as acetylation. Acetylation of salicylic acid produces aspirin.

- Reactions involving cleavage of carbon-oxygen (C–O) bond in alcohols
Reaction with hydrogen halides:ROH + HX ➡ R–X + H2OThe difference in reactivity of three classes of alcohols with HCl distinguishes them from one another (Lucas test).
- Reaction with phosphorus trihalides: Alcohols are converted to alkyl bromides by reaction with phosphorus tribromide
- Dehydration: Alcohols undergo dehydration (removal of a molecule of water) to form alkenes on treatment with a protic acid e.g., concentrated H2SO4 or H3PO4, or catalysts such as anhydrous zinc chloride or alumina.

Ethanol undergoes dehydration by heating it with concentrated H2SO4 at 443 K.

Secondary and tertiary alcohols are dehydrated under milder conditions. For example

Mechanism
- Formation of protonated alcohol.

- Formation of carbocation: It is the slowest step and hence, the rate-determining step of the reaction.

- Formation of ethene by elimination of a proton.

The acid used in step 1 is released in step 3. To drive the equilibrium to the right, ethene is removed as it is formed.
Oxidation:
Such a cleavage and formation of bonds occur in oxidation reactions. These are also known as dehydrogenation reactions as these involve loss of dihydrogen from an alcohol molecule.


Strong oxidising agents such as acidified potassium permanganate are used for getting carboxylic acids from alcohols directly. CrO3 in anhydrous medium is used as the oxidising agent for the isolation of aldehydes.

A better reagent for oxidation of primary alcohols to aldehydes in good yield is pyridinium chlorochromate (PCC), a complex of chromium trioxide with pyridine and HCl.

Secondary alcohols are oxidised to ketones by chromic anhydride (CrO3).

When the vapours of a primary or secondary alcohol are passed over heated copper at 573 K, dehydrogenation takes place and an aldehyde or a ketone is formed while tertiary alcohols undergo dehydration.

Chemical Properties of Phenols
Electrophilic Substitution Reactions
- Nitration:

The ortho and para isomers can be separated by steam distillation. o-Nitrophenol is steam volatile due to intramolecular hydrogen bonding while p-nitrophenol is less volatile due to intermolecular hydrogen bonding which causes the association of molecules.

With concentrated nitric acid, phenol is converted to 2,4,6-trinitrophenol. The product is commonly known as picric acid. The yield of the reaction product is poor.

Halogenation:

When phenol is treated with bromine water, 2,4,6-tribromophenol is formed as white precipitate.

Kolbe’s reaction

Reimer-Tiemann reaction

Reaction of phenol with zinc dust

Oxidation

Some Commercially Important Alcohols
Methanol
Methanol, CH3OH, also known as ‘wood spirit’, was produced by destructive distillation of wood.

Ethanol

Preparation of Ethers
1. By dehydration of alcohols

The formation of ether is a nucleophilic bimolecular reaction (SN2) involving the attack of alcohol molecules on a protonated alcohol, as indicated below:

Williamson synthesis

Ethers containing substituted alkyl groups (secondary or tertiary) may also be prepared by this method. The reaction involves SN2 attack of an alkoxide ion on primary alkyl halide.

Better results are obtained if the alkyl halide is primary. In case of secondary and tertiary alkyl halides, elimination competes over substitution. If a tertiary alkyl halide is used, an alkene is the only reaction product and no ether is formed. For example, the reaction of CH3ONa with (CH3)3C–Br gives exclusively 2-methylpropene.

Phenols are also converted to ethers by this method. In this, phenol is used as the phenoxide moiety.

Physical Properties

Both ethoxyethane and butan-1-ol are miscible to almost the same extent i.e., 7.5 and 9 g per 100 mL water, respectively while pentane is essentially immiscible with water. Can you explain this observation? This is because just like alcohols, oxygen of ether can also form hydrogen bonds with water molecule as shown:

Chemical Properties of Ethers
- Cleavage of C–O bond in ethers

Alkyl aryl ethers are cleaved at the alkyl-oxygen bond due to the more stable aryl-oxygen bond. The reaction yields phenol and alkyl halide.

Ethers with two different alkyl groups are also cleaved in the same manner.

The order of reactivity of hydrogen halides is as follows:
HI > HBr > HCl. The cleavage of ethers takes place with concentrated HI or HBr at high temperature.
Mechanism



However, when one of the alkyl group is a tertiary group, the halide formed is a tertiary halide.


Electrophilic substitution

Halogenation:


Friedel-Crafts reaction:

Nitration:

Unit VIII: Aldehydes, Ketones, and Carboxylic Acid
The general formulas of these classes of compounds are given below:


Aldehydes, ketones and carboxylic acids are widespread in plants and animal kingdoms. They play an important role in the biochemical processes of life. They add fragrance and flavour to nature, for example, vanillin (from vanilla beans), salicylaldehyde (from meadow sweet) and cinnamaldehyde (from cinnamon) have very pleasant fragrances.

Nomenclature of Aldehydes
(a) Common names

Alkyl phenyl ketones are usually named by adding the name of acyl group as prefix to the word phenone. For example

(b) IUPAC names
Other aromatic aldehydes are hence named as substituted benzaldehydes.




Structure of the Carbonyl Group

The high polarity of the carbonyl group is explained based on resonance involving a neutral (A) and dipolar (B) structures as shown.

Preparation of Aldehydes and Ketones
By oxidation of alcohols
Aldehydes and ketones are generally prepared by oxidation of primary and secondary alcohols, respectively
By dehydrogenation of alcohols
This method is suitable for volatile alcohols and is of industrial application. In this method, alcohol vapours are passed over heavy metal catalysts (Ag or Cu). Primary and secondary alcohols give aldehydes and ketones, respectively
From hydrocarbons
(i) By ozonolysis of alkenes: As we know, ozonolysis of alkenes followed by reaction with zinc dust and water gives aldehydes, ketones or a mixture of both depending on the substitution pattern of the alkene.
(ii) By hydration of alkynes: The addition of water to ethyne in the presence of H2SO4 and HgSO4 gives acetaldehyde. All other alkynes give ketones in this reaction
Preparation of Aldehydes
From acyl chloride (acid chloride)/Rosenmund reduction.

From nitriles and esters/Stephen reaction

Alternatively, nitriles are selectively reduced by diisobutylaluminium hydride, (DIBAL-H) to imines followed by hydrolysis to aldehydes:

Similarly, esters are also reduced to aldehydes with DIBAL-H.

From hydrocarbons
By oxidation of methylbenzene
Strong oxidising agents oxidise toluene and its derivatives to benzoic acids. However, it is possible to stop the oxidation at the aldehyde stage with suitable reagents that convert the methyl group to an intermediate that is difficult to oxidise further. The following methods are used for this purpose.
(a) Use of chromyl chloride (CrO2Cl2)/Etard reaction.


By side chain chlorination followed by hydrolysis

By Gatterman – Koch reaction

Preparation of Ketones
From acyl chlorides

From nitriles

From benzene or substituted benzenes/ Friedel-Crafts acylation reaction.

Physical Properties
The following compounds of molecular masses 58 and 60 are ranked in order of increasing boiling points.

The lower members of aldehydes and ketones such as methanal, ethanal and propanone are miscible with water in all proportions because they form hydrogen bonds with water.

Chemical Reactions
Since aldehydes and ketones both possess the carbonyl functional group, they undergo similar chemical reactions.
Nucleophilic addition reactions
Contrary to electrophilic addition reactions observed in alkenes, the aldehydes and ketones undergo nucleophilic addition reactions.


(ii) Reactivity
Aldehydes are generally more reactive than ketones in nucleophilic addition reactions due to steric and electronic reasons. Sterically, the presence of two relatively large substituents in ketones hinders the approach of nucleophiles to carbonyl carbon than in aldehydes having only one such substituent. Electronically, aldehydes are more reactive than ketones because two alkyl groups reduce the electrophilicity of the carbonyl carbon more effectively than in former.
Some important examples of nucleophilic addition and nucleophilic addition-elimination reactions:
(a) Addition of hydrogen cyanide (HCN):

(b) Addition of sodium hydrogensulphite:

(c) Addition of alcohols:

(d) Addition of ammonia and its derivatives:



Reduction
(i) Reduction to alcohols:
Aldehydes and ketones are reduced to primary and secondary alcohols respectively by sodium borohydride (NaBH4) or lithium aluminium hydride (LiAlH4) as well as by catalytic hydrogenation
(ii) Reduction to hydrocarbons
The carbonyl group of aldehydes and ketones is reduced to CH2 group on treatment with zincamalgam and concentrated hydrochloric acid [Clemmensen reduction] or with hydrazine followed by heating with sodium or potassium hydroxide in high boiling solvent such as ethylene glycol (Wolff-Kishner reduction).

Oxidation
Aldehydes differ from ketones in their oxidation reactions. Aldehydes are easily oxidised to carboxylic acids on treatment with common oxidising agents like nitric acid, potassium permanganate, potassium dichromate, etc. Even mild oxidising agents, mainly Tollens’ reagent and Fehlings’ reagent also oxidise aldehydes.

Ketones are generally oxidised under vigorous conditions, i.e., strong oxidising agents and at elevated temperatures. Their oxidation involves carbon-carbon bond cleavage to afford a mixture of carboxylic acids having lesser number of carbon atoms than the parent ketone.

(i) Tollens’ test:

(ii) Fehling’s test:

(iii) Oxidation of methyl ketones by haloform reaction:

Reactions due to a-hydrogen
Acidity of α-hydrogens of aldehydes and ketones:

(i) Aldol condensation:

(ii) Cross aldol condensation:


(i) Cannizzaro reaction:

(ii) Electrophilic substitution reaction:

Carboxylic Acids
Carbon compounds containing a carboxyl functional group, –COOH are called carboxylic acids.
Carboxylic acids may be aliphatic (RCOOH) or aromatic (ArCOOH) depending on the group, alkyl or aryl, attached to carboxylic carbon. A large number of carboxylic acids are found in nature. Some higher members of aliphatic carboxylic acids (C12 – C18) known as fatty acids, occur in natural fats as esters of glycerol.
Nomenclature


Structure of Carboxyl Group
In carboxylic acids, the bonds to the carboxyl carbon lie in one plane and are separated by about 120°.

Methods of Preparation of Carboxylic Acids
1. From primary alcohols and aldehydes

2. From alkylbenzenes

3. From nitriles and amides

4. From Grignard reagents

5. From acyl halides and anhydrides

6. From esters

Physical Properties

Chemical Reactions
Reactions Involving Cleavage of O–H Bond
Acidity
Reactions with metals and alkalies

Carboxylic acids dissociate in water to give resonance-stabilised carboxylate anions and hydronium ions.

For convenience, the strength of an acid is generally indicated by its pKa value rather than its Ka value.
pKa = – log Ka
Effect of substituents on the acidity of carboxylic acids:

The effect of the following groups in increasing acidity order is
Ph < I < Br < Cl < F < CN < NO2 < CF3
Thus, the following acids are arranged in order of increasing acidity
(based on pKa values):

Direct attachment of groups such as phenyl or vinyl to the carboxylic acid, increases the acidity of corresponding carboxylic acid, contrary to the decrease expected due to resonance effect shown below:

This is because of greater electronegativity of sp2 hybridised carbon to which carboxyl carbon is attached. The presence of electron withdrawing group on the phenyl of aromatic carboxylic acid increases their acidity while electron donating groups decrease their acidity.

Reactions Involving Cleavage of C–OH Bond
1. Formation of anhydride

2. Esterification

Mechanism of esterification of carboxylic acids:

3. Reactions with PCl5, PCl3 and SOCl2

4. Reaction with ammonia


Reactions Involving –COOH Group
1. Reduction

2. Decarboxylation/Kolbe electrolysis

Alkali metal salts of carboxylic acids also undergo decarboxylation on electrolysis of their aqueous solutions and form hydrocarbons having twice the number of carbon atoms present in the alkyl group of the acid. The reaction is known as Kolbe electrolysis
Substitution Reactions in the Hydrocarbon Part
1. Halogenation/Hell-Volhard-Zelinsky reaction.

2. Ring substitution

Unit IX: Amines

Structure of Amines

Classification

Nomenclature Of Alkylamines and Arylamines



Preparation of Amines
- Reduction of Nitro Compounds

- Ammonolysis



- Reduction of Nitriles

- Reduction of Amides

- Gabriel Phthalimide Synthesis


- Hoffmann Bromamide Degradation Reaction

Physical Properties


Chemical Properties of Amines
- Basic character of amines


- Basicity in terms of Kb and pKb values


- The order of basic strength in case of methyl-substituted amines and ethyl-substituted amines in aqueous solution is as follows:

- Alkanamines versus ammonia


- Arylamines versus ammonia

On the other hand, anilinium ion obtained by accepting a proton can have only two resonating structures (kekule).

- Acylation


Amines also react with benzoyl chloride (C6H5COCl). This reaction is known as benzoylation.

Unit X: Biomolecules
Carbohydrates General Formula, Cx(H2O)y
Classification of Carbohydrates
- Monosaccharides

Structure of Glucose
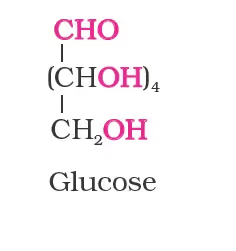
- the molecular formula was found to be C6H12O6
- On prolonged heating with HI, it forms n-hexane, suggesting that all six carbon atoms are linked in a straight chain.

- Glucose reacts with hydroxylamine to form an oxime and adds a molecule of hydrogen cyanide to give cyanohydrin. These reactions confirm the presence of a carbonyl group (>C = O) in glucose.

- Glucose gets oxidised to six-carbon carboxylic acid (gluconic acid) in reaction with a mild oxidising agent like bromine water. This indicates that the carbonyl group is present as an aldehydic group.

- Acetylation of glucose with acetic anhydride gives glucose pentaacetate which confirms the presence of five –OH groups. Since it exists as a stable compound, five –OH groups should be attached to different carbon atoms.

- On oxidation with nitric acid, glucose as well as gluconic acid both yield a dicarboxylic acid, saccharic acid. This indicates the presence of a primary alcoholic (–OH) group in glucose.

The exact spatial arrangement of different –OH groups was given by Fischer after studying many other properties. Its configuration is correctly represented as I. So gluconic acid is represented as II and saccharic acid as III.
- Glyceraldehyde contains one asymmetric carbon atom and exists in two enantiomeric forms

(+) Isomer of glyceraldehyde has a ‘D’ configuration
- The structure of glucose and glyceraldehyde is written in a way that most oxidised carbon (in this case –CHO)is at the top.

Cyclic Structure of Glucose

- The two cyclic hemiacetal forms of glucose differ only in the configuration of the hydroxyl group at C1, called anomeric carbon (the aldehyde carbon before cyclisation). Such isomers, i.e., α-form and β-form, are called anomers. The six-membered cyclic structure of glucose is called pyranose structure (α– or β–), in analogy with pyran.

Structure of Fructose

- Furan is a five-membered cyclic compound with one oxygen and four carbon atoms.

- The cyclic structures of two anomers of fructose are represented by Haworth structures as given.

Disaccharides
Sucrose:


laevorotation of fructose (-92.4°) is more than dextrorotation of glucose (+ 52.5°), the mixture is laevorotatory.
Maltose:

Lactose:

Polysaccharides
Starch:
It is a polymer of a-glucose and consists of two components–Amylose and Amylopectin.


Cellulose:

Glycogen:
Molecular formula - (C6H10O5)n

Fisher Model

Proteins
Amino Acids

- All naturally occurring amino acids are in the L-series in which the –NH2 group on the left and –OH group on the right as L-glyceraldehydes.

Nomenclature of Amino Acids




Classification of Amino Acids
- Essential Amino Acids

- Non-essential Amino Acids

Peptide Linkage of Amino Acids and their Classification

Secondary structure of proteins:

β-Pleated sheet structure of proteins

Protein Structure (Two Subunits Of Two Types In Quaternary Structure)

Primary, secondary, tertiary, and quaternary structures of haemoglobin

Enzymes

Classifications of Vitamins



Chemical Composition of Nucleic Acids

- DNA contains four bases viz. adenine (A), guanine (G), cytosine (C) and thymine (T). RNA also contains four bases, the first three bases are the same as in DNA but the fourth one is uracil (U).


Structure of Nucleic Acids–Nucleoside and Nucleotide

Formation of a dinucleotide

Simplified Version of Nucleic Acid Chain

Double-strand helix structure for DNA

Structural difference between DNA and RNA

Importance of Organic Chemistry CBSE Class 12 Formulas
The organic chemistry portion contributes 31 marks in the final examination thus, to ace the Class 12 board exams, organic chemistry formulas and reaction sheets are needed. By using the Organic Chemistry CBSE Class 12 Formulas, students can benefit in the listed ways.
- Understanding the organic chemistry formula sheet can help students predict the outcome when the involved reactants under/without conditions react to form a product.
- The formula sheet can help students determine the distinct isomeric forms and their properties. Formulas are important to understand the functional groups in organic chemistry, their nature, and their acidic-basic properties.
- Formulas are essential to infer the compound structure by its name and to determine the synthesis sequence of the reaction. It will help recognize and name compounds as per the IUPAC standards.
How to Use this Organic Chemistry Formula Sheet?
Students often find themselves wondering about how this product was formed and what the reactants were in the first place. This chemistry section requires a slightly different approach, unlike the other two. To start preparing for organic chemistry IUPAC, chemical reactions, and other formulas in Class 12, students need to
- Understand the basics of general organic chemistry.
- Starting with it can help in understanding the reaction mechanism writing.
In this formula list, students can find the reactions and their mechanisms, reaction mechanisms like substitution and elimination reactions. The chapter-wise reactions and applications given in one place can help in quick revision and exam preparation.

.svg)










.avif)











